orange book pharmacy definition
Before understanding different drug ratings it is necessary. This book contains the list of all drugs approved in the United States as safe.

The Introduction Of An Orange Book
Available on-line as the Electronic Orange Book EOB it.

. The updates incorporate changes made after the UKs exit from the European Union on the 31 January 2020. As it is still published in a book with an orange cover the nick-name of the Orange Guide remains today. RPS Member Price 6150.
Preface to Approved Drug Products with Therapeutic Equivalence Evaluations Orange Book provides info on how the book came to be relevant terms and codes user responsibilities and more. The Orange Book Introduction. Then use the Ingredient Search for.
Get emails about this page. The Orange Book is a compendium of significant unimplemented nonmonetary recommendations for improving departmental operations. 1 Need of the Orange Book Definition Introduction to 2 History 3 the Orange Book Objectives 4 3 Contents of the Orange Book 5 18 4 Cumulative Supplement 19 5.
The 2022 edition of Rules and Guidance for Pharmaceutical Manufacturers and Distributors the Orange Guide is now available through MedicinesComplete. Providing you with a single authoritative source of European and UK guidance. Office of Generic Drugs Policy Center for Drug Evaluation Research US.
The Orange book has been revised. Recent research from PharmacyChecker shows that 88 of the most commonly. For more information on the Orange Book including its history see the Orange Book Preface.
It now contains more than just GMP with sections covering both UK and EU legislation on pharmaceuticals so it is not just a guide as well as Good Distribution Practice and Active. Updated with Orange Book. This determines the ingredient s.
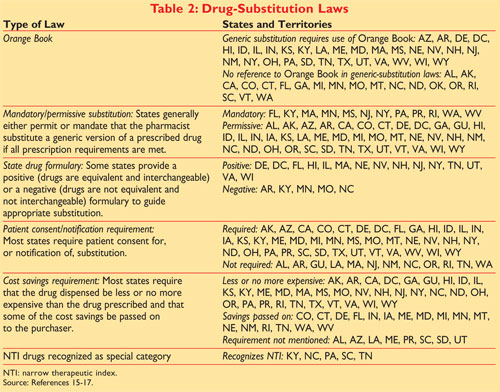
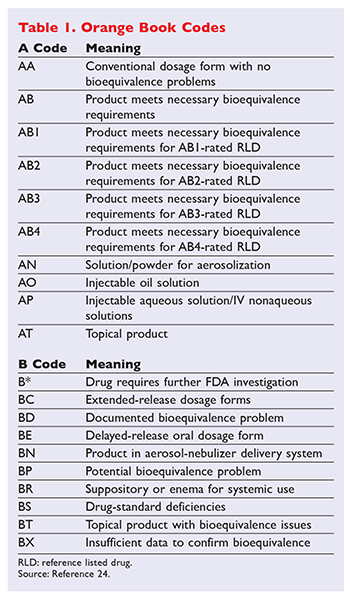
1 Generic substitution laws are state specific and many require use of the Orange Book. The orange book is a list of generic drugs approved by FDA. The orange book consist of five main sections.
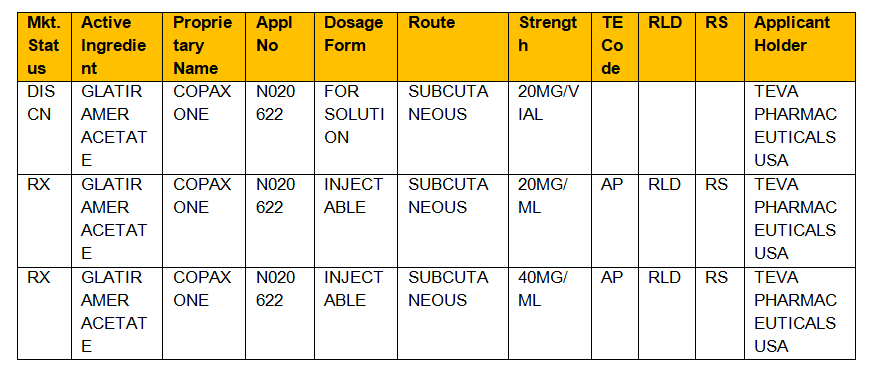
Formally known as Approved Drug Products with Therapeutic Equivalence Evaluations the orange book lists drugs which are not only safe but also effective for human use. In the electronic Orange Book a reference standard is identified by RS in the RS column. See answer 1 Best Answer.
The Orange Book Risk Management Principles. First if you have the trade name search the Electronic Orange Books Rx or OTC section using the Proprietary Name search. The FDAs Orange Book identifies approved drug products FDA has draft guidance explaining that certain currently marketed drug ingredients were marketed before current FDA legislation.
Book was published in October 1980 with orange cover and thus the name orange book. Format is RX OTC DISCN. Enforce State Pharmacy Boards Oversight of Patient Counseling Laws.
Rules and Guidance for Pharmaceutical Manufacturers and Distributors 2022 The MHRA Orange Guide MHRA Medicines and Healthcare products Regulatory Agency The MHRA Orange Guide is the essential reference for all manufacturers and distributors of medicines. Sponsors using these products should consult FDA about the need for an IND. Objectives What is a Generic Drug.
Search the Orange Book Database. Because prices can vary significantly from pharmacy to pharmacy. The Orange Book formally titled Approved Drug Products With Therapeutic Equivalence Evaluations is a comprehensive list of approved drug products published by the FDAIt is widely accepted as the authoritative source for determining therapeutic equivalence among multisource drug products.
G o v e r n a n c e and L e a d e r s I n te g ra o n h i p C o l a b or ti o n Information Insight Insight Information Communication. Indias largest online marketplace for automobiles Droom has announced expansion of the scope of Orange Book Value popularly known as OBV to give the fair market value of used mobile phones to the customers. He and others suggest you take the time to comparison shop in the US.
Type The group or category of approved drugs. CDR Kendra Stewart RPh PharmD. Basics in drug approval process with reference to the Orange Book Presented by.
The Orange Book is an important publication published by the FDA that serves as the gold standard reference for generic drug substitution. Levitt also recommends asking your doctor if there is a viable therapeutic alternative or a lower-cost generic drug. The Orange Book is a reference source that gives insight on whether or not two drugs have Therapeutic Equivalences.
The orange book is available in electronic format Electronic Orange Book to provide access to information such as. R i s k r e. An introduction a how to use section the drug product lists appendices and a patent and exclusivity information addendum.
The orange book is published annually and the 2015 edition is 35th edition of orange book1 It is freely available for download and it has search options available on website. Rucha Pathak Roll No. The full publication title is Approved Drug Products with Therapeutic Equivalence Evaluations but it is commonly known as the Orange Book.
Search approved drug products by active ingredient proprietary name. 65 Strengthen FDA. New Delhi India Dec 6 ANI.
Food and Drug Administration. The Office of Inspector General. The book was published again in 1997 2002 and then 2007.
Food and Drug Administration 10903 New Hampshire Avenue Silver Spring MD 20993 1-888-INFO-FDA 1-888-463-6332 Contact FDA.

Pharmaceutical Marketing Plan Process

Pharmacy Prescription Process Definition Steps Knowles Wellness

Investigational New Drug Orange Book Understanding On 505 B 2 A

The Introduction Of An Orange Book
Insights Into Effective Generic Substitution
Regulatory 101 Drug Name Modifiers Definition Categories Generics And Capa Ivt

The Introduction Of An Orange Book

The Introduction Of An Orange Book

The Poetry Pharmacy Returns More Prescriptions For Courage Healing And Hope By William Sieghart
Orange Book And Its Applications Legal Advantage

The Introduction Of An Orange Book
Appendix A Medical Terminology And Abbreviations In Manual For Pharmacy Technicians

/doctor-826e0c116cd549d98e327f1184c622d9.jpg)